The natural occurrence of OH in olivine
Gregory H. Miller, George R. Rossman
Division of Geological and Planetary Sciences
California Institute of Technology, Pasadena, CA 91125, USA
George E. Harlow
Department of Mineral Sciences
American Museum of Natural History
New York, NY 10024-5192
Abstract
Polarized IR spectra of olivine single crystals from
17 different localities show a tremendous variabilty in both mode and
abundance of hydroxide (OH) incorporation. Kimberlitic
olivines contain the most OH at an estimated concentration level of 976
H/106 Si whereas olivines from basalts contain the least at
3 H/106 Si. Olivines of metamorphic and hydrothermal
origin have widely varying concentration levels between those of
basalts and kimberlites. Over 30 distinct OH absorption bands have been
identified. Most of these bands are not unique to individual localities
but may be found in samples from several different localities.
Pleochroism is consistent among localities, but relative band
intensities vary. No evidence is found for molucular H2 in
olivine.
Hydrous minerals have been identified in olivine by
their characteristic OH absorption bands. Serpentine is commonly
found and is clearly distinguishable from intrinsic OH. Talc is
present in only one sample. Prominent OH bands at 3572 and 3525 cm-
1 are attributed to humite-group minerals.
San Carlos, Arizona, olivines annealed in the
presence of H2O, develop absorption bands which are found in
natural samples; however, the OH absorption spectra of these annealed
olivines are not identical to those of any single natural
crystal. Sharp-band OH abundances in annealed samples are an
order of magnitude lower than the max. measured in natural
specimens. The mechanical properties determined from these
annealed olivines may not be directly applicable to mantle olivine
because both the OH sites and concentrations are different.
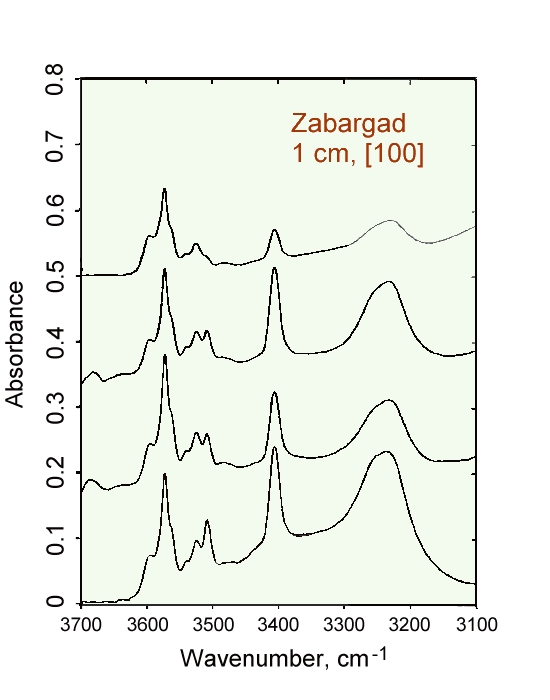
Spectra of olivines from Zabargad Island polarized in the [100]
direction showing the variability observed for individual localities.

