John M. Hughes
Department of Geology, The University
of Vermont, Burlington, VT 05405, U.S.A.
John Rakovan
Department of Geology, Miami University, Oxford,
Ohio 45056,
U.S.A.
Andreas Ertl
Institut für Mineralogie und
Kristallographie, Geozentrum, Universität Wien, Althanstrasse 14, 1090 Vienna,
Austria
George R. Rossman
Division of Geological and Planetary Sciences, California
Institute of Technology, Pasadena,
California 91125-2500
Ivan Baksheev
Geology Department, Lomonosov Moscow State
University, Vorobiovy Gory, Moscow 119992, Russia
Heinz-Jürgen Bernhardt
Institut für Geologie, Mineralogie und Geophysik, Ruhr-Universität Bochum, D-44801 Bochum, Germany
Abstract
The tourmaline atomic arrangement
is one of the last rock-forming silicate structures to be investigated in
detail. Although putatively possessing hexagonal R3m symmetry,
reports of optically-anomalous tourmaline are common, and recently an
occurrence of triclinic tourmaline was reported with dissymetrization resulting
from non-equivalency of the occupants of the tourmaline Y-sites. We here
report the atomic arrangement of a Ni-bearing tourmaline from the Middle Urals, Russia,
in non-conventional triclinic space group R1 (R = 4.41%) to
facilitate comparison with the conventional R3m cell. The
dissymetrization occurs as a result of inequalities of the hexagonal Y
and Z tourmaline sites, in contrast to the single previous structure
study. The atomic arrangement demonstrates that the tourmaline atomic arrangement
is robust, and is capable of incorporating various substituents by modifying
the putative structure in lower symmetries, suggesting that further exploration
of tourmaline’s role in trace-element variation is warranted.
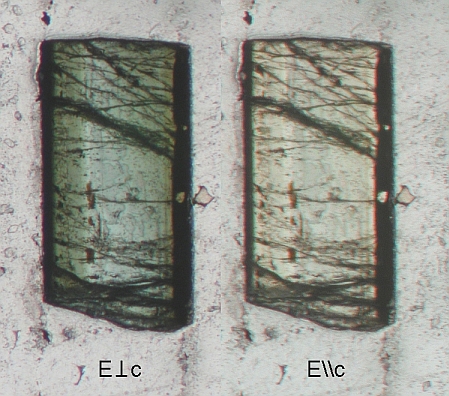
Optical studies
demonstrate a 2V greater than zero. Spectroscopic studies show the optical
absorption spectrum of the Ni-tourmaline has strong absorptions in the 400,
600-700, and 1100 nm regions, in
addition to OH features near 1450, 2300, and 2700 nm. It is concluded that the
color in the E perpendicular to c polarization comes dominantly from Fe
mixed-oxidation-state couples, and from Cr3+. Contributions from the
nickel are believed to be minor and will fall in the regions of strong Cr and
Fe absorption.