 |
 |
Fe2+- and Mn2+-rich tourmalines were used to test whether Fe2+ and Mn2+ substitute on the Z site of tourmaline to a detectable degree. An Fe-rich tourmaline from a pegmatite from Lower Austria was characterized by crystal-structure determination, chemical analyses, and Mössbauer and optical spectroscopy. The sample has large amounts of Fe2+ (~2.3 apfu), and substantial amounts of Fe3+ (~1.0 apfu). On basis of the chemical data, the structural refinement and the spectroscopic data, an initial formula was determined by assigning the entire amount of Fe3+ and Ti4+ to the Z site and the amount of Fe2+ and Fe3+ from delocalized electrons to the Y-Z ED doublet (delocalized electrons between Y-Z and Y-Y):
X(Na0.9Ca0.1) Y(Fe2+2.0Al0.4Mn2+0.3Fe3+0.2) Z(Al4.8Fe3+0.8Fe2+0.2Ti4+0.1) T(Si5.9Al0.1)O18 (BO3)3 V(OH)3 W[O0.5F0.3(OH)0.2]
with a = 16.039(1), c = 7.254(1) Å. This formula is consistent with lack of Fe2+ at the Z site, apart from that occupancy connected with delocalization of a hopping electron. The formula was further modified by considering two ED doublets to yield:
X((Na0.9Ca0.1) Y(Fe2+1.8Al0.5Mn2+0.3Fe3+0.3) Z(Al4.8Fe3+0.7Fe2+0.4Ti4+0.1) T(Si5.9Al0.1)O18 (BO3)3 V(OH)3 W[O0.5F0.3(OH)0.2] .
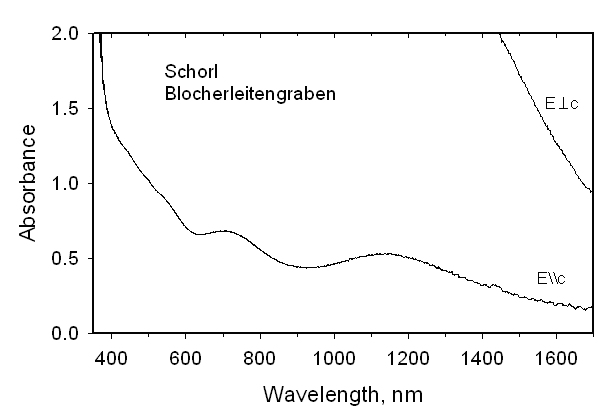
This formula requires some Fe2+ (~0.3 apfu) at the Z site, apart from that connected with delocalization of a hopping electron. Optical spectra were recorded from this sample as well as from two other Fe2+-rich tourmalines. If Fe2+ were to occupy two different 6-coordinated sites in significant amounts and if these polyhedra have different geometries or metal-oxygen distances, bands from each site should be observed. However, even in high-quality spectra we see no evidence for such a doubling of the bands. We conclude that there is no final proof for Fe2+ at the Z site, apart from that occupancy connected with delocalization of hopping electrons involving Fe cations at the Y and Z sites. A very Mn-rich tourmaline from a pegmatite on Elba Island, Italy was characterized by crystal-structure determination, chemical analyses and optical spectroscopy. The optimized structural formula is
X(Na0.6[]0.4) Y(Mn2+1.3Al1.2Li0.5) ZAl6 TSi6O18 (BO3)3 V(OH)3 W[F0.5O0.5] V(OH)3W[F0.5O0.5]
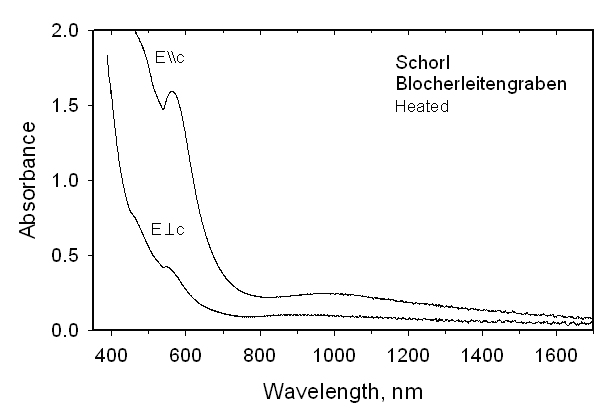
with a = 15.951(2), c = 7.138(1) Å. Within a 3sigma error there is no evidence for Mn occupancy at the Z site by refinement of Al <-> Mn. We conclude that there is no final proof for Mn2+ at the Z site. Oxidation of these tourmalines at 700-750 °C and 1 bar for 10-72 h converted Fe2+ to Fe3+ and Mn2+ to Mn3+ with concomitant exchange with Al of the Z site. The refined ZFe content in the Fe-rich tourmaline increased by ~40% relative to its initial occupancy. The refined YFe content was smaller and the <Y-O> distance was significantly reduced relative to the unoxidized sample. A similar effect was observed for the oxidized Mn2+-rich tourmaline. Simultaneously, H and F were expelled from both samples as indicated by structural refinements and optical absorption spectra. The final species after oxidizing the Fe2+-rich tourmaline is buergerite. Its color had changed from blackish to brown-red. After oxidizing the Mn2+-rich tourmaline, the previously dark yellow sample was very dark brown-red, as expected for the oxidation of Mn2+ to Mn3+. The unit-cell parameter a decreased during oxidation whereas the c parameter showed a slight increase.
 |
 |