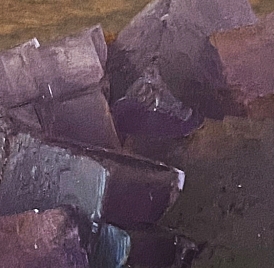1
Ariel University, Physical Department, Ariel, Israel
2
Energy
Geosciences Division, Lawrence Berkeley National Laboratory MS 74-316C,
One Cyclotron Road Berkeley CA United States
3 Division
of Geological and Planetary Sciences, California Institute of
Technology,
Pasadena, California 91125, United States
4 Institut
Lumière Matière UMR 5306, Université Lyon 1 – CNRS, Université de Lyon,
69622 Villeurbanne, France
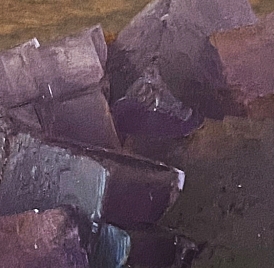

Purple fluorite from Berbes, Asturias, Spain, in daylight (left) and under UV illuination (right)
Click on the images for larger images.
This type of high Na fluorite fades under LWUV but can be brought back with SWUV.
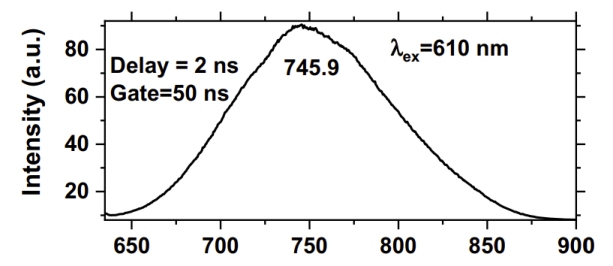
Room Temperature luminescence spectrum of the red emission band
of naturally irradiated fluorite from Mapimi, Durango, Mexico.