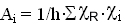Ge 214: Pyroxene homework series: part II
Energy states of Fe2+ in C2v symmetry in the M2 site
Now identify and list the symmetry elements that are preserved. The symmetry elements of Oh are listed on the top of the character table for Oh.
From the character tables, we need to look at the character of T2g and Eg states in Oh and see into what they factor in C2v.
In other words, what linear combination of the rows in the C2v character table to we need to add together to get the characters for the T2g entry?
You should have the following character table for the elememts of Oh that remain in C2v. This is the reducible representation.

which gives the number times the aith irreducible representation occurs in a reducible representation.
h = the order of the group = the number of elements in the group.
e.g. in C2v, h = 1 E + 1 C2 + 1 sigma(h) + 1 sigma(d) = 4
XR = the character of the reducible representation
e.g. for E, XR = 3
Xi = the character of the ith irreducible representation.
e.g. in C2v, for E, Xi = 1
So, the number of A1 states in C2v that derive from T2g is
A1 = 1/4×[3×1 + 1×1 + (-1)×1 + 1×1] = 4/4 = 1
After doing this for all the possible states, you will determine the states into which T2g and Eg split in C2v.
But, you will still not know the energies of the states. We need to go experimental data at this point.
We will use a low-iron enstatite where iron in the M(2) site dominates the spectrum.
The Experimentally Determined Energy states of Fe2+ in C2v symmetry.
The first figure 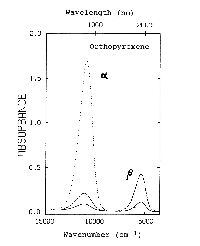 shows the alpha (dots), beta (dash) and gamma (solid line)
spectra of
a 100 micrometer thick
bronzite (Fs14) in the visible and near-infrared
range.
shows the alpha (dots), beta (dash) and gamma (solid line)
spectra of
a 100 micrometer thick
bronzite (Fs14) in the visible and near-infrared
range.
The second figure 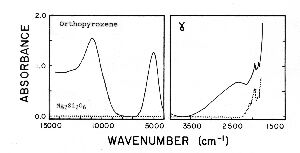 shows only the gamma spectrum of two 0.5 mm thick crystals
in the visible through the mid-infrared range. The solid line is for
hypersthene (Fs39.5), and the dotted line is for
synthetic enstatite (Fs0.00).
shows only the gamma spectrum of two 0.5 mm thick crystals
in the visible through the mid-infrared range. The solid line is for
hypersthene (Fs39.5), and the dotted line is for
synthetic enstatite (Fs0.00).
Compile a list of band positions and polarizations of all Fe2+ bands (both in terms of the indicatrix direction and in terms of the morphological axes). Also determine the molar absorptivity (epsilon parameter) for each band.
The Origin of Polarizations and Pleochroism.
1) Electronic transitions, in general are polarized. They correspond to a redistribution of electronic charge in three-dimensional space. These redistributions of electrons have vector properties and must match the electric dipole of the exciting radiation if there is to be effective coupling of energy between the incident radiation and the electron cloud.
Here is picture of two orthopyroxene crystals that show the visible-wavelength anisotropy when viewed in linerally polarized light. As you can see from the spectra above, the anisotropy is much more pronounced in the near-infared region.
We want to know the polarizations of transitions such as:
That is to say, we want to know along which C2v
molecular axis
(or axes) a particular electronic transition (such as A1 ->
B1) will occur. Once we know this, we need only
to align a linear polarizer in this direction to make the transition
occur (this assumes that
we know the orientation of the molecular axes with respect to the
morphological
axes of the crystal).
Quantum mechanics tells us the intensity of an electronic transition will be proportional to an integral of the form:
![]()
![]()
m is an operator that corresponds to the components of an electric dipole having the form:
m = (k1 • X + k2 • Y + k3 • Z)
We want to evaluate integrals of the form:
![]()
From purely symmetry considerations, we can tell if the integrals are nonzero. This is analogous to evaluation of integrals of the odd (sine) and even (cos) functions.
Assume for a moment that the A1 state is the ground state. (Actually, ground states are often A1 states) We need to evaluate a whole series of integrals of the type:
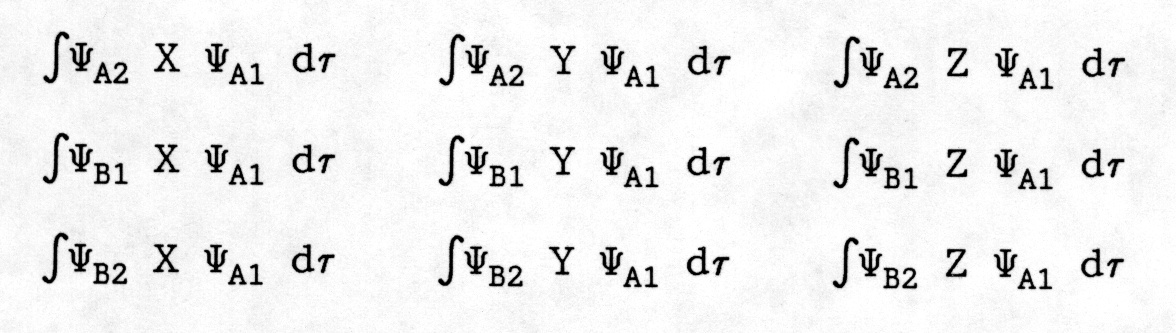
Where A1, B1, A2, and B2 are the designations for all the possible states.
Because A1, B1, A2, and B2 are not only designations for the
possible states
but are also the designation for their symmetry, we can forget the
details
of the (complicated) mathematical descriptions of the wavefunctions,
and
can instead cnocentrate ONLY on the symmetry properties.
Thus we need to evaluate integrals of the type:
From the character table, we learn that the molecular z axis in C2v has the symmetry Al. (You can work this out by going through all the symmetry operations with the axis [+ and - lobes, don’t forget] or you can read it directly from the character table; the letter z in the Al row indicates that anything which is mathematically described by a function first order in just z [such as the z axis which is, after all, just +z] has Al symmetry.)
Thus, we need to evaluate integral (1) which has components with the symmetry property:
Is this integral non-zero?
Here is how we proceed:
We need to work out a multiplication table for C2v
wherein we
multiply all possible symmetries by each other. Take the case of
A2 • Al • Al
We can do this with pairs of functions: (A2 • Al) • Al
This answer is equivalent to A2. In fact, you can show that Al times anything else is the something else.
Our problem of evaluating (A2 • Al) • Al has now been reduced to one of evaluating (A2) • Al
We already know the answer to this is A2.
In order for the integral to be non-zero, the integrand must be invarient under the operation of the symmetry elements for the group. This demands that the totally symmetric representation (Al) must be a component of the integrand. (This comes back to the even, odd function problem; we now are just worried about it in three dimensions).
Thus, we can now conclude that the transition from an Al state to an A2 state will not occur if it is stimulated with linearly polarized light vibration in the direction of the molecular Z axis.
Now work out the answer for all three components of a possible transition from a) an Al state to an A2 state.
b) an Al state to a Bl state.
c) an Al state to a B2 state.
As you can now see, only one polarization component is allowed for each of these possible transitions.
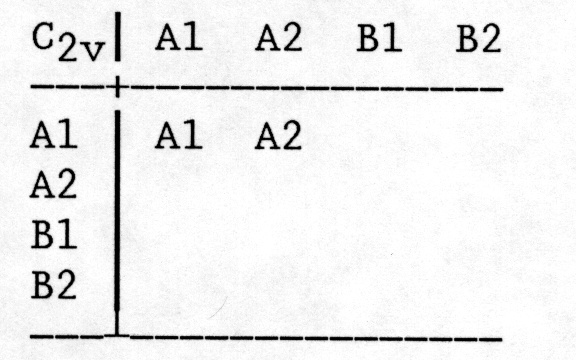
2) Work out the multiplication table for all possible pairs of symmetry elements. Fill in the rest of the table
This will allow you to fill in the remaining entries in the following table which states the polarization directions for all possible transitions in C2v.
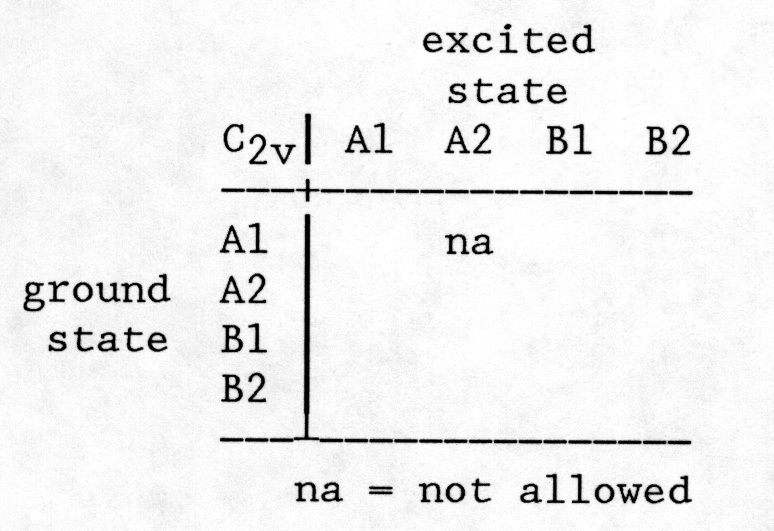
Our final step will be interpret the experimental data on the basis of our theoretical modeling.
Comparison of Models to Real Data
1) Notice that the highest energy absorption band
is polarized in the a
direction of the indicatrix. Use your projections
of the crystal structure to establish which molecular axis of the C2v
M(2) site is oriented exactly on (or at least close to) the a direction.
2) Now use your table of selection rules (polarizations) from above to list all possible transitions which are polarized along the molecular axis closest to the a indicatrix direction. These are the candidates for the transition: ground state to the highest excited state. Can you establish which transition is the actual one in these orthopyroxenes? (Hint, the answer is yes.) Do it.
3) Once you have identified the states involved in the highest energy transition, you have established the ground state of Fe2+ in this site. From the polarization data you should be able to assign the remaining experimentally observed transitions to particular excited states. Construct an energy level diagram for Fe2+ in the M(2) site where the energy scale in linear and to scale, where the states are identified, and the polarization rules indicated for each state.
4) We have one state left over and
unaccounted for. This
remaining state has not yet been experimentally detected. It would be
buried under intense infrared vibrational modes that dominate the
infrared spectrum. You can read about where some
theoretical calculations predicted it should be in the article by
Goldmann
and Rossman (1977) The spectra of iron in orthopyroxene revisited: the
splitting
of the ground state. American Mineralogist, 62, 151-157.
Another source for some more information on the spectroscopy of pyroxenes is in the Reviews of Mineralogy series, Volume 7, CT Prewitt, editor, Pyroxenes, chapter 3, Pyroxene Spectroscopy