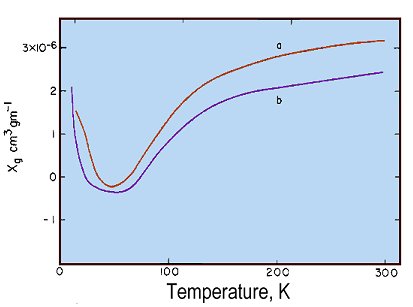
Gram magnetic susceptibilities of enH2[(FeHEDTA)2O]ˇ6H2O (a) and Na4[(FeEDTA)2O]ˇ12 H2O (b) as a function of temperature
| Harvey J. Schugar1, George R.
Rossman2, Collin G. Barraclough3, Harry B.
Gray2 1Rutgers University 2California Institute of Technology 3University of Melbourne |
Magnetic susceptibility results and extensive electronic, infrared, and Mossbauer spectral data are presented for enH2[(FeHEDTA)2O]ˇ6H2O, Na4[(FeEDTA)2O]ˇ12 H2O, FeHEDTAˇ H2O, and NaFeEDTAˇ3H2O (HEDTA = N-hydroxoethylethylenediaminetriacetate, EDTA = ethylenediaminetetraacetate, and enH22+ = ethylenediammonium cation). The magnetic and spectral data establish an electronic structural model for the oxo-bridged dimers in which pairs of S = 5/2 Fe(III) ions interact antiferromagnetically, with J ≈ -95 cm-1. The oxo-bridged dimers show marked intensity enhancement of the one-center Fe(III) ligand-field bands. There are also several uv bands which are interpreted as arising from simultaneous electronic excitations of Fe(III) pairs. A simple high-spin ligand-field model modified by spin-spin interaction is judged to be considerably more appropriate than the Dunitz-Orgel molecular orbital approach as a vehicle for describing oxo-bridged Fe(III) dimers.
