Magnetic susceptibility of the iron hydroxy sulphates and chromates
| George R.
Rossman Division of Geological and Planetary Sciences California Institute of Technology Pasadena, CA 91125 |
Optical and infrared absorption and magnetic susceptibility data are
reported for the ferric iron hydroxy
sulfate minerals and compounds: basic iron sulfate, Fe(OH)SO4;
butlerite,
Fe(OH)SO4·2H2O;
parabutlerite, Fe(OH)SO4·2H2O;
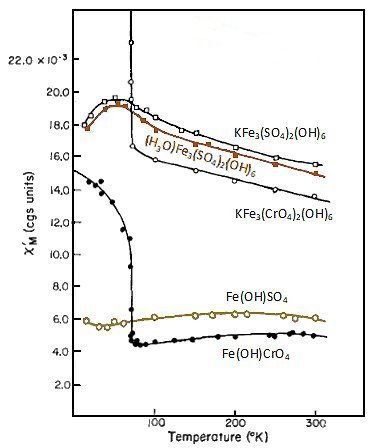
fibroferrite, Fe(OH)SO4·5H2O; jarosite, KFe3(SO4)2(OH)6;
and stewartite, MnFe2(H2O)6(OH)2(PO4)2.
The relationships among intensity of color, indices of refraction,
pleochroism, antiferromagnetic interaction among Fe3+ ions,
and structure are discussed for 7A
corner-linked chain structures and the two-dimensional Fe3+
sheets of the jarosites, For all of these compounds the greatest
intensity of the Fe3+
absorption bands and the highest refractive
index occur when the vibration direction of the incident light is
aligned with the cation chains or in the plane of the jarosite sheets.
The ligand-field absorption
band intensities of all of these compounds are enhanced up to two
orders of magnitude
above what they are in magnetically dilute compounds.
