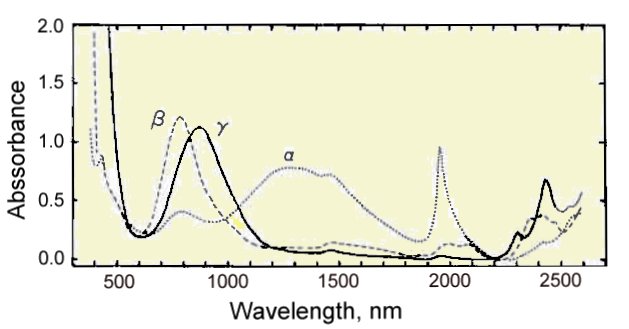
Optical absorption spectrum of 0.150 mm thick guildite crystals.
| Che'ng
Wan, Subrata Ghose Department of Geological Sciences University of Washington Seattle, Washington 98195 |
George R.
Rossman Division of Geological and Planetary Sciences California Institute of Technoogy Pasadena, CA 91125 |
