Edwin Schauble, George R. Rossman, and Hugh P.
Taylor, Jr.
Division of Geological and Planetary Sciences
California Institute of Technology
Pasadena, CA 91125
Geochemica Cosmochemica Acta 65, 2487-2497
Abstract
The
magnitude and direction of equilibrium iron-isotope (54Fe
- 56Fe) fractionations among simple iron-bearing
complexes and a-Fe metal are
calculated using a combination of force-field modeling and
existing infrared, Raman, and inelastic neutron scattering
measurements of vibrational frequencies. Fractionations of up to
several per mil are predicted between complexes in which iron is
bonded to different ligands (i.e. 4 per mil for [Fe(H2O)6]3+
vs. [FeCl4]- at 25 ºC). Similar
fractionations are predicted between the different oxidation
states of iron. The heavy iron isotopes will be concentrated in
complexes with high-frequency metal- ligand stretching
vibrations, which means that 56Fe/54Fe will
be higher in complexes with strongly bonding ligands such as CN-
and H2O relative to complexes with weakly bonding
ligands like Cl- and Br-. 56Fe/54Fe
will also usually be higher in Fe(III) compounds than in
Fe(II)-bearing species; the Fe(II) and Fe(III) hexacyano
complexes are exceptions to this rule of thumb. Heavy iron
isotopes will be concentrated in sites of 4-fold coordination
relative to 6-fold coordination. Model results for a ferrous
hexacyanide complex, [Fe(CN)6]4-,
are in agreement with predictions based on Mössbauer spectra (
Polyakov, 1997), suggesting that both approaches give reasonable
estimates of iron-isotope partitioning behavior.

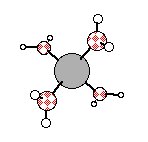
Here are animations of the Raman-active stretch in Li(H2O)4+ and the Raman-active stretch in Fe(H2O)63+. They are calculated with the ab initio package GAMESS at the Hartree-Fock level using the fairly large 6-311+G(d) basis set for tetraquo Li+ and with a triple-zeta valence basis set (of unknown quality) for hexaaquo Fe3+. The frequency for the Li-O stretch is within ~3% of experimental observations. The predicted Fe-O stretch appears to be too low by ~10%, and the predicted bond length is too long by ~0.05 A.