The Crystal Chemistry of Kornerupine. II. The Role of Hydrogen
Mark A. Cooper and Frank C.
Hawthorne
Department of
Geological Sciences, University of Manitoba Winnipeg, Manitoba, Canada R3T 2N2
Luisa Ottolini
CNR-Centro di Studio per la Cristallochimica
e la Cristallografia
(CSCC)
via Abbiategrasso,
209, 1-27100 Pavia, Italy
Edward S. Grew
Department of
Geological Sciences, University of Maine, Orono,
ME 04469, U.S.A.
T. Kurtis Kyser
Department of
Geological Sciences, Queen's University
Kingston, Ontario, Canada K7L 3N6
George R. Rossman
Division of
Geological and Planetary Sciences
California Institute of Technology,
Pasadena, CA 91125 U.S.A.
ABSTRACT
The H content of a suite of well-characterized samples of
kornerupine-prismatine minerals, ([],Mg,Fe) (Al,Mg,Fe)9 (Si,Al,B)5 O21
(OH,F), has been measured by KFT (Karl Fischer Titration) and HLE (Hydrogen-Line Extraction). The KFT results show (OH
+ F) < 1.0 apfu (atoms per formula unit) and the deviation from 1.0 (OH + F) apfu is negatively correlated withthe Fe content of kornerupine, whereas the HLE results show (OH + F) = 1.0 apfu. Crystalchemical considerations
suggest that the HLE results are correct. The anomalously low KFT results are
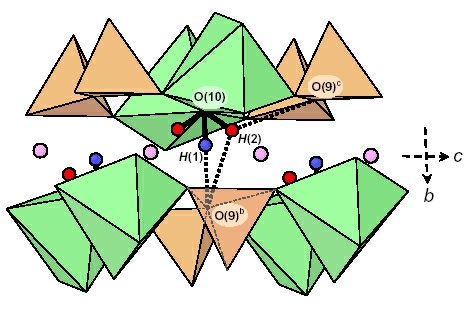
due to incomplete breakdown of the sample and only partial release of the constituent H. Detailed examination of the electron density around the
O(10) (= OH) site in several kornerupine crystals showed two distinct H sites, H(1) and H(2); the H(1) site occurs onthe (001) mirror plane, and the H(2) site occurs off
the (001) mirror plane. The H atom is usually disordered off the (001) mirror plane (at z = 1/4) at the H(2) site. The latter is only ~1.1 Å from the X site, and hence
mutual occupancy of locally adjacent H(2) and X sites is not possible; this constraint gives the maximum possible occupancy of the X site as (1 + F)/2 apfu. When H occupies the H(1) site on the
(001) mirror plane, both adjacent X
sites
must be locally vacant. Polarized infrared spectra in the principal (OH)-stretching region show
fine structure that can be assigned to specific short-range arrangements of cations around the
O(10) [= (OH), F] site involving occupancy of the X
site
by divalent cations or vacancies, adjacent T(1) sites by Si–Si or Si–Al, adjacent M(1) sites by Mg or
Fe2+, and occupancy of the H(1) or H(2) sites by H or [].
Canadian Mineralogist: 47, 263-274.
